Day 2 :
- Chemistry Education | Biochemistry | Nanoscience & Materials Chemistry | Organic & Inorganic chemistry | Polymer Chemistry
Location: Tromso

Chair
Jerry P. Suits
University of Northern Colorado, USA

Co-Chair
Gloria Quintanilla
Universidad de Alcalá, Spain
Session Introduction
Jerry P. Suits
University of Northern Colorado, USA
Title: Conceptual Assessment of Learning Outcomes from Animations and Simulations

Biography:
Jerry Suits completed his MS in biochemistry from Texas State University (1976) and PhD in science education at the University of Texas at Austin (1985). He has been the editor of a book on the pedagogic roles of animations and simulations in chemistry courses (2013). He has published more than 20 papers in peer-reviewed journals and book chapters. He and his graduate students have presented more than 100 papers at national (USA) and international conferences. He has been serving as an editorial board member of the Journal of Computers in Mathematics and Science Teaching.
Abstract:
Manabu Sumida
Ehime University, Japan
Title: Changing Trends in Chemistry Research Modes and the Imperative Needs of Chemistry Education for Gifted Students

Biography:
Manabu Sumida is Professor of science education at the Ehime University in Japan. He holds a BA in chemistry from Kyushu University and PhD in science educadtion from Hiroshima University. He was a visiting researcher at the University of Georgia in 1998 and visiting scholar at the University of Cambridge in 2012. He has been Director of Kids Academy Science (a special science programme for gifted young children) for 9 years. He is currently Director of Japan Society for Science Education, and Regional Representative for Asia of the International Council of Association for Science Education.
Abstract:
Chemistry has been experiencing dramatic changes since the beginning of the twentieth century. The enterprise is diverse and complex, and it involves international collaboration. However, the trends in modern chemistry do not seem to be reflected in education, although many children show a strong interest in natural phenomena from an early age and demonstrate an outstanding ability to think creatively and in abstract terms. This presentation summarises the global trends in Nobel Laureates in Chemistry from 1901 to 2012 in order to illustrate how giftedness in chemistry is a requirement for the new century. This presentation proposes different chemistry education modes for gifted students in formal and informal settings. Some of Ehime University’s special gifted education programmes, which deal with identification, curriculum development, practice, and assessment, are introduced as case examples of chemistry education for the gifted. This presentation also analyses the effects of special education programmes and discusses the similarities and differences between their effects on gifted students and regular students. The development of chemistry curricula and teaching materials that accommodate the special needs of gifted students and the implementation of related teaching methods and assessments are relevant to all teaching subjects, school types, and education in general. Moreover, they can be used in the educational activities of communities and societies around the world.
Karin Larsson
Uppsala University, Sweden
Title: Electronic Properties of Various B-Doped Diamond (111)//Dye Molecule Interfaces

Biography:
Karin Larsson is a Professor in Inorganic Chemistry at the Dept. of Chemistry-Ångström Laboratory, Uppsala University, Sweden, and Guest Professor at the University of Science and Technology Liaoning, China. In addition, Karin Larsson is elected member of IVA (Royal Swedish Academy of Engineering Sciences), division V (Mining and Materials;Vice-Chair). Prof. Karin Larsson is today the head of the Theoretical Materials Chemistry Group at the Div. of Inorganic Chemistry, Dept. of Chemistry-Ångström laboratory. The scientific focus is on interpretation, understanding and prediction of the following processes/properties for both solid/gas interfaces, as well as for solid/liquid interfaces; i) CVD growth (e.g. diamond, BN, and graphene), iii) interfacial processes for renewable energy applications (e.g. electrochemical processes), and iv) bio-functionalization of surfaces (e.g. bone regeneration).
Abstract:
Diamond is a widely known material for its many excellent properties (eg., high thermal conductivity, high breakdown voltage, transparency, chemical inertness and bio-compatibility). A B-doped diamond is an excellent p-type material for solar cell usage. Due to some specific properties (e.g., large chemical inertness, very high carrier mobility for both electron and holes, high transparency), it is considered as one of the strongest candidates for photovoltaic electric generation. However, in order to implement the usage of diamond in solar energy applications, properties like the i) electrochemical window, ii) possibility for interfacial charge transfer, and iii) stability of functionalized surface, have to be further studied and optimized.
In the past investigation, the adsorption of different dye molecules onto H-terminated diamond (111) surfaces, have been theoretically studied using Density Functional Theory (DFT) calculations under periodic boundary conditions. The diamond surfaces were B-doped in order to make them p-type semi-conducting. The choice of dyes was based on the match between the electronic structures of these H-terminated B-doped diamond surfaces, and the respective dye molecules. The dye molecules in the present study include C26H13NO3S4 (A), C35H37NO2S3 (B), C34H38OS2 (C), C32H36OS2 (D), and C31H35S3Br (E). These dyes differ in the various functional groups, which have the role as electron acceptors. The main goal with the present study was thereby to investigate and compare the photovoltaic efficiency of the various dyes when attached to B-doped and H-terminated diamond (111) surfaces. Of a special interest was to study the i) absorption spectra of the dye, ii) degree of electron transfer over the diamond//dye interface, iii) electron transfer arte, iv) electron-hole recombination, and v) diamond//dye bond strength.
The calculated absorption spectra for in principle all of the different dyes were shown to be located in the most intense part of the sunlight spectrum. For the E dye, the spectrums were more positioned towards the UV light range. The usage of a combination of these different dyes would hence be an optimal choice in order to improve the light harvesting in a photovoltaic process.
Furthermore, the calculations identified the LUMO’s for the B, C and D dyes to be positioned on the upper end of the molecules, which also will be the position of the electron acceptor when being excited by light. For dyes A and E, there were though certain extensions of the LUMO’s to the lower end of the molecules (i.e., towards the diamond surface), which will also increase the electron-hole recombination rates.
Calculation of electron transfer was to ensure that the HOMO of these dyes were positioned at a lower energy compared to the upper edge of the valence band of the B-doped diamond surface. Moreover, all dyes were found to bind with strong C-C covalent bonds to the diamond (111) surface.
Kimberly B. Fields
University of South Florida, USA
Title: STEM Education Abroad: Yes you can!
Time : 14:00-14:30

Biography:
Kimberly B. Fields has completed her PhD at the age of 35 years from University of South Florida. She is an instructor in chemistry with eight years of experience in higher education. She has published 7 papers in peer-reviewed journals and has written two book chapters. Current research interests are in chemical education with an emphasis on organic chemistry and a key member of the USF Science in Florence program development team.
Abstract:
The University of South Florida (USF), with an undergraduate enrollment of 31,000 students, has a vibrant study abroad program involving hundreds of students, dozens of faculty, and over 60 countries. We have developed and administer a STEM study abroad program called USF Science in Florence which is centered in Florence, Italy. Traditionally, study abroad programs do not include chemistry, physics, or physician observation/comparative health care systems courses – that are completely in line with the students’ STEM major. In our study abroad program, Organic Chemistry I & II, Physics I with Calculus, and General Biochemistry are taught with the same rigor as when those courses are taught during the standard semester. This affords STEM majors the ability to stay on track for their respective degrees while experiencing study abroad. The study abroad experience is viewed positively by prospective employers and post-graduate program selection committees. Our STEM study abroad program is called Science in Florence and is centered in Florence, Italy.
The class sizes are small (30 students or less) with many more opportunities for individualized instruction and support than the typical USF on-campus experience. The courses offered in Florence, usually are high-enrollment courses (200-300 students per lecture) when taught in Tampa, FL. Field learning excursions to reinforce course learning objectives are incorporated as much as possible. Tours to local establishments take place weekly and include Officina Profumo – Farmaceutica di Santa Maria Novella, the Museo Galileo, and Fattoria di Maiano. Discussion of relevant scientific topics dovetail nicely with topics in chemistry. Feedback from students involved are overwhelmingly positive of the program
Andreas Schedy
University of Education Freiburg, Germany
Title: Graphene – exciting insights into the synthesis and chemistry of the miracle material of the 21st century and its implementation in chemistry lessons for the first time
Time : 14:30 -15:00

Biography:
Andreas Schedy studied from 2011 to 2016 at the University of Education Freiburg for teaching at secondary schools in the subjects of chemistry, mathematics and geography and graduated in 2016 with the first state examination. Since July 2016 he has been doing his doctorate in the working group of Prof. Dr. Marco Oetken at the University of Education Freiburg, funded by a doctoral scholarship. As part of his doctoral thesis, Mr. Schedy deals with the experimental development of the topic Graphene.
Abstract:
The two-dimensional modification of carbon which is also known as “graphene” is an extremely interesting material due to its physical properties, such as a very high electrical conductivity and an intrinsic mechanical strength that is even better than that of steel. For that reason, it has already been called the “miracle material“ of the 21st century. Up to now, the synthesis of graphene was to dangerous to implement it into the school curricula. The authors present a method of synthesizing graphene which can be put into practice at school without hesitation. In addition, the authors present some experiments with which the varying properties of graphene oxide and graphene during synthesis can be shown. In this way, different structure property relationships, which appear to be one of the most important concepts in chemistry lessons, can be analyzed. The authors also present a field of application for graphene
GLORIA QUINTANILLA
Universidad de Alcalá, Spain
Title: Weekly Reflection Papers: Development of the strategy
Time : 15:00-15:30

Biography:
Mª Gloria Quintanilla López became Titular Professor in Organic Chemistry at the University of Alcalá in 1985. Her teaching task is focussed mainly to the students of Health Biology, Chemistry and Pharmacy. As researcher she has dealt with Organic Synthesis and Electrochemistry. She is Erasmus Coordinator for the Chemistry Faculty since 1989 and organized the Choir of the University for the last 39 years. In the last ten years she is the coordinator of a teaching innovation group of the University of Alcalá based in the “Reflection activity”.
Abstract:
All social networking such as Facebook, Twitter or WhatsApp produce an immediacy which prevents, especially the young people, from going deeper in any thought or communication among others. As a contrast, a group of teachers of different subjects of the University of Alcalá, headed and caught up by an Organic Chemistry professor, have implemented a teaching strategy in which the main objective is the reflection, named “Weekly Reflection Papers”, (WRP). Initially in this experience, the students handed in to the lecturer every week their “weekly assignments”, in which they schematically express the most important ideas related to the topic presented during the classes of the previous week including also their impressions or comments on those aspects which they found especially difficult or interesting. Teachers give back to the students the corrected WRP and respond to the student’s comments. The implemented strategy represents an excellent vehicle to allow us to explore and analyse the learning process.
However, we checked that the reflective ability of students had to be enhanced, consequently in this communication we describe how along the years we have put more emphasis in the way the reflection has to be done helped by questions as: “What is the most important contribution given to you by the professor in order to increase your personal development? What changes have been produced in your intellectual baggage as a consequence of the classes? Which are the most difficult aspects you have found in your learning process? What can you suggest to improve the teaching and learning process? Where can you find in your real life the knowledges you have recently acquired?”
Mian Jiang
University of Houston Downtown, USA
Title: Embedding petrochemical/nuclear components into analytical curricula: the chemical STEM bridging
Time : 15:50-16:20
Biography:
Mian Jiang completed his PhD from Wuhan University and research associate studies from Northwestern University and New Mexico State University respectively. He currently teaches analytical chemistry at the University of Houston Downtown. He has published more than 50 papers in peer-reviewed journals, and served in various professional capacities in his institution and fields.
Abstract:
Petrochemical components were recently incorporated into our curricular efforts. The main driving force lies at the societal relevance of a sustainable analytical chemistry curriculum. Crude oil exploration, processing, and production of basic, intermediate, and final petrochemicals, all bear the natures of material and characterization studies. They are in line with the University’s high-impact education mission and one of the local economic focuses (as the world’s energy capital). Despite of the natural match or extension between analytical sciences and petro-industry, few reports in this curricular incorporation were published thus far. We started with the incorporation of petrochemical experiments into undergraduate research and STEM workshops. These directly address a wide spectrum of petroleum/petrochemical industry and study. The experiments, designed and tailored to reflect material science and characterization means, included the crude oil viscosity measurement (addressing the up-stream exploration sector), corrosion quantitation (for the mid-stream pipeline or transportation), and preliminary polymer production and characterization (for down-stream end-user products). These ideas were also tested in the existing analytical chemistry classes for teaching effectiveness. Initial responses were positive, with the rising enrollments and motivation to learn. Some students showed interests to deepen these practice into Problem-based learning (PBL) after regular labs. Our current activities concentrate on the embedding of non-aqueous (petroleum liquid) titration for chemical analysis, and characterization of hydrocarbon-bearing rocks and carcinogen petrochemicals (benzene, toluene, ethylbenzene, and xylene, BTEX) in instrumental analysis. Our efforts in updating analytical curriculum are preliminary and forward-looking. They have the potential to improve learning in both material and characterization studies, and may expand into other chemistry disciplines or interdisciplinary fields.
Arnos Arshaki Hovhannisyan
Scientific-Technological Center of Organic and Pharmaceutic Chemistry, Armenia
Title: Polymerization in Monomer - Water Heterogeneous Systems
Time : 16:20-16:50

Biography:
Arnos Arshaki Hovhannisyan received his doctorate at the age of 31 from the Moscow Technical Institute of Fine Chemical Technologie. He is Doctor of Chemical Sciences, Professor. He is head of Laboratory of Polymer Dispersions. He published more than 100 works in well-known journals and a monograph titled "The Theory of Emulsion Polymerization".
Abstract:
Polymerization in monomer - water heterogeneous systems has special properties different from those of other methods of radical polymerization. These properties are detected when the polymerization is conducted in highly disperse systems, or what is common to be considered as emulsion polymerization (EP).
The main components of emulsion polymerization are the monomer, water, emulsifier and initiator. Usually emulsifier molecules don’t participate in the elementary acts of polymerization; however, the rate and degree of EP are increased simultaneously when the concentration of the emulsifier is increased.
There are many theories and models that describe the mechanism of emulsion polymerization. In some of these, the authors introduce the emulsifier concentration in the kinetic equations of radical polymerization. These models and theories are briefly discussed in this presentation.
The main purpose of this report is to represent the results of several experimental studies that show a possible link between the specifics of EP and physical and chemical processes occurring at monomer-water interfaces. In these experiments the polymerization is carried out without the use of emulsifier under static conditions in styrene-water, vinyl acetate-water and chloroprene-water systems, from which is clearly visible the topological picture of dispersed particles generation in a narrow interface of the monomer – water system.
M Gloria Quintanilla
University of Alcalá, Spain
Title: Pooling of ideas after writing the individual weekly refl ection paper: Collaborative refl ection

Biography:
Abstract:
Silvana Claudia Caglieri
National Technological University, Argentina
Title: Theoretical Study of Pentyl Alcohol Acetylation
Biography:
Abstract:
Lucila Giammatteo
FES Cuautitlán UNAM Posgrado MADEMS – QuÃmica, Mexico
Title: Assessing chemistry laboratory skills through a competency-based approach in a high-school chemistry course

Biography:
Lucila Giammatteo studied pharmaceutical and biological chemistry at Universidad Nacional Autónoma de México. She has been working as a chemistry teacher specialized in multicultural education at Instituto Tecnológico de Estudios Superiores de Monterrey for the past 4 years. She is currently studying a Master's degree in Chemistry Teaching at Universidad Nacional Autónoma de México.
Abstract:
Given the changes with the last educational reform in Mexico, most high-schools have changed to a competency-based model. Since there is a huge gap between the instruction and the evaluation process, there is an evident need to homologate both. This paper proposes a novel strategy to evaluate the competencies which are related to laboratory practices, such as formulating hypothesis, systematizing information, applying security norms, handling residues, collecting data, contrasting results in an experiment and communicating conclusions.
Experimental sciences specific competencies were selected and evaluated with the use of an assessment tool where the students' levels of achievements were described considering the curriculum and the graduate's profile. A random sample of students averaged 16 years old was chosen. After submitting the laboratory report, Students' feedback was delivered through Blackboard platform in prompt time, so the students could take those comments into consideration and improve their performance in the following practices. A total of 7 practices were evaluated in this manner during a semester in an inorganic chemistry high-school course. The topics were chosen in such a way that those seen in theoretical classes were reinforced with the laboratory practices.

Biography:
Roberto Acevedo, PhD is nowadays an independent researcher, retired (last April, 2017) and fully engaged in research in three main areas: Basic and Applied Technology, Entrepreneurship and Social Business, Scientific Psychology and Higher Education. In his academic career, he was promoted to full Professor in both Universidad de Chile (UCh) and Universidad Mayor (UM). He was appointed in a variety of positions as academician and administrator, in relevant positions, among others, such as: Dean of the Faculty of Engineering (UM), Head of Research and Development (UM), Member of the Senate (UCh), Member of Faculty Council (Facultad de Ciencias Físicas y Matemáticas. UCh), Head of the Departamento de Química (Facultad de Ciencias Físicas y Matemáticas. UCh). As for his studies are concerned, he became a Licenciado en Filosofía con mención en Química (Magister) at the Universidad de Chile (1974), PhD at both Birkbeck and King´s Colleges. University of London (1981), Held a post doctorial position at the Chemistry Department. Charlottesville. University of Virginia, USA (1982). Some of his academic distinctions are as follows: post doctorial position and fellowship at the University of Virginia, USA (1982), Honorary Research Fellow. University of London. UK (1984, 1985, 1990, 1991), Bursary of the EC. DG-XII (1991), Invited Speaker. Advanced Study Institute. NATO-ASI. Riva del Sole, Italy (1988), Visiting Professor at the City University of Hong Kong (1986).
Roberto Acevedo has focused his attention in both lecturing to a broad number of students in different topics and also in research in areas such as: Linear and non Linear Optics in New Inorganic Luminescent Materials of the Rare Earths, Dynamics of Crystals, Cooperative Effects, Jahn - Teller (Static and dynamics), Pseudo Jahn - Teller in Coordination Compounds of the Transition Metal and Lanthanide Complex Ions, Scientific Psychology, Advanced Studies in Higher Education, Social Business and Entrepreneurship. He has been the supervisor of a substantial number of students: 12, working for their academic degrees of MSc, DSC, and 78 working for their Professional Titles. His outcome in research may be divided into sections: (a) National Publications: 117, (b) Publications in International Congress: 42, (c) Publications in electronic journals: 148 (d) International Publications (ISI): 85 and Author of 17 Books. Many Conferences delivered in Chile and Abroad.
Abstract:
In a number of research studies, we have dealt with a number of attributes which are of paramount importance to improve the quality of life for any well-organized society. The main target in this set of studies is to both focus and underline the importance of the knowledge in the state of the art. It is crucial to realize that nowadays broad topics such as Robotic, Artificial Intelligence, Data mining, Nano science, Nanotechnology and Biotechnology are the basis for any development to be achieve in the short, medium and long term. We do recognize the importance of these broad and essential areas of knowledge and development but it must be admitted that as a country, we have not done the work properly and in due time. The offset with reference to developed countries is most significant. It is rather straightforward to conclude that our efforts in these areas are not enough to overcome these difficulties. Chile is sad example of a country, where highly qualify professionals are, in number, either scarce or non-existing. We believe that we must develop and increase economical resources on a regular basis, so as attributes such proactivity, novel ideas, entrepreneurship and social business among others are indeed to be considered seriously. Chile has considerable resources, such as Cupper, Lithium and Rare Earths and a dessert with clean skies and sun light (so this is without any doubt a rather unlimited supply of solar energy). Only scarce efforts have been dedicated to obtain benefit of these free sources. There are also, a number of other traditional resources such as a significant and large forest, wine and other commodities. A better analysis of both our strength and weak points should be taken into account and people should be seduced to work for the country welfare. It is obvious the role to be played by a high level training in subjects such as Chemistry and Biology in addition to Mathematics, Physics, Computing, will be essential to have available a reasonable number of competent professional working for the productive sector.
Manal Y Sameeh
Umm al Qura university - allith collage , KSA
Title: Synthesis of antifungal compound from Isolated curcumin
Biography:
Manal Sameeh is the head of chemistry department in Allith College (branch of Umm Al-Qura University). She has completed her PhD at the age of 31years from king Abdul-Aziz University in natural products and photochemistry. She achieved her postdoctoral studies from Umm Al-Qura University. Her scientific interests are natural chemistry, photochemistry and biochemistry; she has published many papers in reputed journals. She is a member in Saudi Chemical Society and the Arab Science and Technology Foundation. She has attended many national and international conferences.
Abstract:
Turmeric belongs to the ginger family and is a rhizomatous herbaceous perennial plant . it may be considered the strong effective nutritional supplement in existence . It has revealed numerous medical studies about the importance of turmeric in the treatment of a large number of illnesses beginning of cancers, leading to Alzheimer disease. Turmeric is the spice which gives curry yellow color.
This study investigates the best methods to isolate, purify, and identify the chemical composition and the important biological activity of turmeric. At the beginning , the turmeric was bought from market and followed by:
- The plant used in research was classified as Turmeric (Curcuma Longa L.)
- The percentage of moisture in the dried samples was assessed. The results showed that the moisture content of turmeric tubers was 12%
- Various compounds of dry turmeric rhizomes were extracted by ethanol, GC-MS was achieved to this extract and showed the presence of a large number of compounds belonging to different types of terpenes and aromatic compounds. The largest component of these compounds was turomene (19%), zingiberen(17%) and curcumene (20%)
- Isolation of curcumin from ethanolic extract.
- Synthesis of curcumin epoxide by using mcpba thermally.
- Antifungal activity was done to new compound “curcumin epoxide” with the fungi (Candida albican, Aspergillus parasiticus, Fusarium proleferatum, Pencillium verrucosum and Aspergillus niger) and the results were highly positive.
Hari Babu Bollikolla
Acharya Nagarjuna University, India
Title: Synthesis and biological evaluation of Schiff base derivatives of furfural based heterocyclic flavanoids
Biography:
Dr. Hari Babu Bollikolla has completed his PhD at the age of 28 years from Acharya Nagarjuna University. He is the former head of Dept. of chemistry, of University College of sciences. He has published more than 100 papers in reputed journals and has been serving as an editorial board member of reputed journals. He has guided more than ten Ph.D. students.
Abstract:
Synthesized a total of twelve new furfural flavone Schiff base derivatives starting from commercially available phloroglucinol. In the first stage the corresponding acetophenone key intermediate was synthesized from the starting material and was condensed with furfural followed by cyclisation, and formylation. Finally, the formylated flavone was condensed with various aromatic primary amines to obtain the desired Schiff base derivatives. All the synthesized furfural flavone Schiff base derivatives were tested for their anticancer and antibacterial activities and the results were analysed in the light of their structural activity.
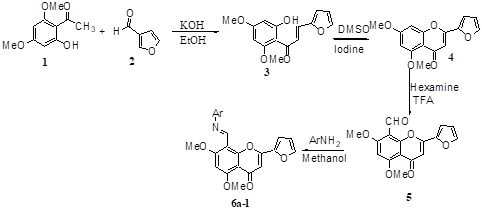
Scheme-1: Synthesis of Schiff base derivatives of furfural flavones
Kiramat Shah
University of Swat, Pakistan
Title: Synthesis and Characterization of supramolecules and its applications as a chemosensor for drugs
Biography:
Kiramat Shah is Assistant Professor at Institute of Chemical Sciences, University of Swat, Swat KPK Pakistan. He has obtained his Ph.D degree from International Center for Chemical and Biological Sciences University of Karachi, Karachi Pakistan. He is synthesizing macrocycles and macromolecules using different advanced reactions particularly Azide -Alkyne Click reaction, Glaser coupling, Sonogashira coupling, amide bond formation using coupling reagents and Mannich reaction. For the characterization of these molecules we use different techniques like 1H NMR, 13C NMR, advanced 2D NMR, EI-MS, ESI-MS, MALDI, UV-vis and FT-IR spectroscopy. To explore the chemo-sensing properties of these macrocycles and macromolecules for different antibiotics, dyes, explosives and metal ions we use UV-visible and Fluorescence spectroscopy.
Abstract:
The monitoring of pharmaceutical drugs in the environment is of great importance word wide. For example, in Karachi Pakistan due to contaminated water six children were died and about 200 were fill ill in 2005. A large number of pharmaceutical drugs in highly alarming amount were found in different components of drinking water (surface water, drainage, and effluent) of Karachi in the microgram-per-liter range during bioassay directed chemical analysis. The photophysical evaluation of supramolecular organic molecules as optical probes for detection of water toxins has been recognized to be very selective, sensitive, and economical as compared to the previously used methodologies.
Synthesis, characterization and molecular recognition properties of fluorene based supramolecular cleft is reported. The cleft molecule was prepared in a single-step with good yield (85% yield), by linking Fluorene with 1-ethyl piperazine. The cleft molecule was carefully characterized using various spectroscopic techniques such as NMR and mass spectrometry. The supramolecular interaction of cleft with amoxicillin, 6APA, aspirin, captopril, cefotaxime, ceftriaxone, cefuroxime, diclofenac, penicillin, and cephradine was evaluated by fluorescent spectroscopy. The molecular recognition studies showed that amoxicillin selectively binds with cleft in the presence of other drugs. The analytical method developed for the supramolecular interaction of molecular cleft and amoxicillin was validated at varying pH, concentration and temperature during recognition process. Job's plots indicated that the stochiometry of the interactions between the cleft and the amoxicillin was 1:1.
Salah Akkal
University of Constantine, Algeria
Title: The challenges facing the teaching of chemistry in general education Algeria
Biography:
Salah Akkal received his PhD. in Organic Chemistry from University of Constantine, Algeria in 2001. He was supervised 4 M.Sc. Theses and 10 Ph.Ds. He is professor in natural product chemistry since 2007 in Chemistry Department, Faculty of science. He has published more than 99 papers in reputed journals and has been serving as an editorial board member of repute and has been serving as a reviewer for many articles in her specialization. He attended more than 30 International Conferences and five workshops. He teaches the general chemistry and natural product courses for the students of Faculty of Science and Faculty of Technology at University of Constantine, Algeria.
Abstract:
Salah Akkal think that it is necessary to link the theoretical side with the practical aspect of chemistry teaching in a way that allows the student to get closer to this understanding, since chemistry touches large areas in our daily life. We do not forget that for students of fine science and technology, their choice of chemistry, Or graduate studies in chemistry after the end of the common trunk, they must have a solid composition in the application of chemistry supports their theoretical balance and allows them to enter the world of chemical industry, pharmaceuticals and petrochemicals.
The student's study of the chemistry experiments also gives rise to the enthusiasm for following up on the observations and conclusions of the experiments of chemistry
One of the current priorities is to give importance to the practical aspect by providing specialized laboratories for this purpose for the first years of university so as to ensure an integrated composition, although this is not easy because of the large number of students in the common trunk, but follow-up and supervision can overcome the various difficulties by forming committees A follow-up aimed at coordinating and creating the appropriate framework to put this reform on the right track and from which to reap the desired results.
Henadzi Filipenka
Grodno branch of the Kazan Research Radio Technology Institute, Belarus
Title: Nature of chemical elements
Biography:
Henadzi Filipenka graduated from the Leningrad Electro technical Institute. He grew monocrystals of iron-yttrium garnet. Worked out technologies of powder metallurgy in the Grodno branch of the Kazan Research Radio Technology Institute. He worked as a teacher of materials science in Grodno Electro technical College.
Abstract:
The main problem is that using X-rays, we have determined the crystal lattices of different materials, and why they are so, and not others are not yet known. For example, copper crystallizes in the fcc lattice, and iron in the bcc, which becomes fcc on heating, this is used for heat treatment of steels. Copper does not change the crystal lattice when heated. There are many factors affecting the crystallization in the literature, so they decided to remove them as much as possible, and the metal model in the article, say so, is ideal, i.e. all atoms are the same (pure metal) without inclusions, without implants, without defects, etc. using the Hall effect and other data on properties, as well as the calculations of Ashcroft and Mermin, my main determining factor for the type of lattice was the core of the atom or ion, which resulted from the transfer of some electrons to the conduction band. It turned out that the metal bond is due not only to the socialization of electrons, but also to external electrons of atomic cores, which determine the direction or type of the crystal lattice. The change in the type of metal lattice can be connected with the transition of an electron to the conduction band or its return from this zone. Phase transition. It is shown that in the general case, the metal bond in the closest packages (hec and fcc) between the centrally chosen atom and its neighbours is presumably carried out by means of nine (9) directional bonds, in contrast to the number of neighbours equal to 12 (twelve) (coordination number). Probably the "alien" 3 (three) atoms are present in the coordination number 12 stereometrically, and not because of the connection. The answer is to give an experimental test.
Sheila Qureshi
Weill Cornell Medical College, Qatar
Title: A Western pedagogy from an Eastern context: Reflections from a trans-national Chemistry Education research project
Biography:
Dr. Sheila Qureshi is a Senior Lecturer in Chemistry at Weill Cornell Medical College in Qatar (WCM-Q). She obtained her B.Sc. degree in Chemistry and Biology from University of London, Goldsmiths College. Dr. Qureshi received her Ph.D. in Synthetic Organic Chemistry from the University of Cardiff where her PhD thesis focused on synthesis of C-glycosides. Her postdoctoral research at the University of Zurich, Switzerland. Later on, she moved to work in the chemical industry where her work involved pharmaceutical production support and development.
Qualified in education as well as research, she uses teaching strategies such as Process Orientated Guided Inquiry Learning (POGIL) and she supports that through action research to maximize the benefit of such pedagogical approaches in Chemistry in the Foundation Program. Currently published 5 papers on this work.
Abstract:
In this paper, we report on outcomes from a recently ended chemistry education research project that facilitated the transition of a western pedagogical practice into a middle-eastern context like Qatar using a curriculum evaluation framework. An inquiry based approach such as Process Oriented Guided Inquiry Learning (POGIL) was implemented in the foundation chemistry course at Weill Cornell Medicine in Qatar for students to develop key process skills such as teamwork, collaboration, and critical thinking, which foster lifelong learning and professional growth in medicine. Student-centered learning introduced at the foundational level can maximize their development, especially in the sciences.
The positive impact of POGIL was evident from students’ improvement of their cognitive and affective characteristics in a trans-national setting thus demonstrating efficacy and cultural transferability of the pedagogy. The study also focuses the effect of POGIL activities on students’ confidence level in understanding of essential key concepts in chemistry. The talk also reflects the chemistry educator’s scholarship of teaching and learning activities related to discipline-based education research that used inquiry-based approaches in Qatar over a 6 year period, 3 of which we funded by the Qatar Foundation.
Muhammad Saeed Ullah
Kocaeli University, Turkey
Title: Optimization of Froth Flotation Variables for Pyritic Sulfur Removal from Pakistan’s Coal (Bituminous)
Biography:
Abstract:
Coals are heterogeneous, complex and noncrystalline macromolecules containing both organic and inorganic materials. Inorganic constituents, especially sulfur plays a decisive role in the utilization of coal systems. The undesirable ash and sulfur contents can be reduced using physical–chemical and chemical methods. ‘Froth flotation’ is a physical–chemical method that is capable of reducing pyritic sulfur and the ash content of coal. In this research work, key operating variable (pH, air flow rate, composition of feed and separation time) were investigated in a lab-scale froth flotation unit and separation time & air flow rate were found to be the most effective variables for the removal of pyrite sulfur and ash contents from the coal samples. Wetting agent (Polyvinyl Alcohol) and frothing agent (Pine oil) are used that helps for stabilizing air bubble in the froth flotation column and capturing the pyrite sulfur from the column. Present research showed that PVA has a good role as good wetting agent for the pyrite sulfur/coal mixture. Considering the market price of PVA, it is the most feasible option as wetting agent. The optimum values for pH, separation time and air velocity were 7, 40 minutes and 90 scfh respectively. At these optimum values removal of sulfur and ash contents were highest. This combined approach consisting of physical followed by chemical cleaning of coal was found to have potential applications for significant removal of ash and sulfur from bituminous coal with less investment and less generation of waste water.
Nader Noroozi Pesyan
Urmia University, Iran
Title: New and an Interesting Epimer and Osazone Charts in Carbohydrate Chemistry
Biography:
Nader Noroozi Pesyan was born in Pesyan village in the suburb of Adjabshir city in East Azerbayjan province in the capital of Tabriz city, the north west of Iran, in 1968. He studied applied chemistry at the Sistan and Baluchistan University (Zahedan), and obtained his B.Sc. degree in 1993 and obtained his M.Sc. in Azad University of Yazd (Yazd) in 1996 and his Ph.D. of Organic chemistry from Isfahan University of Technology (IUT) in 2004 in the group of Professor H. A. Dabbagh at IUT (Isfahan). He is an academic member (professor) in Faculty of Chemistry at Urmia University from 2005.
Abstract:
One of the major classes of substances common to living systems are carbohydrate so these compounds as very familiar to us that we call many of them as sugar [1,2]. Recently, we have reported the new monosaccharide’s barcoding that caused drawing of Fischer projection of the linear monosaccharaides to be easily [3] and this new barcodes forced the invention of the new monosaccharide’s osazone chart [4]. In this educational research a new epimeric chart (Scheme 1, left) introduced for easily determination of the kind of epimers in each monosaccharide using the corresponding barcode. This new epimeric chart is facilitating the determination and the prediction of any kind of epimers in each monosaccharide. Osazone chart (Scheme 1, right) indicated the new determination rule for finding of a pair aldose and a ketose that make the same osazone.
- Chemistry Education | Biochemistry | Polymer Chemistry
Location: Tromso

Chair
Eva Trnova
Masaryk University, Czech Republic
Session Introduction
Manabu Sumida
Ehime University, Japan
Title: Enquiry for Innovation in a Primary Science Classroom - A Pilot Study Focusing on a Lesson on ‘Dissolving’ in Grade 5
Time : 9:30-10:00

Biography:
Manabu Sumida is Professor of science education at the Ehime University in Japan. He holds a BA in chemistry from Kyushu University and PhD in science educadtion from Hiroshima University. He was a visiting researcher at the University of Georgia in 1998 and visiting scholar at the University of Cambridge in 2012. He has been Director of Kids Academy Science (a special science programme for gifted young children) for 9 years. He is currently Director of Japan Society for Science Education, and Regional Representative for Asia of the International Council of Association for Science Education.
Abstract:
The 21st century is the era of ‘a knowledge-based society’ where new knowledge, information and technology are becoming increasingly important for activities in all walks of life, and science is the driving force. The purpose of this study was to examine the contemporary science lessons in primary science classrooms. A pilot study was conducted in a grade 5 regular science classroom related to the process of ‘dissolving’. The pilot lesson had three stages: 1) sharing scientific language, 2) practicing scientific methods and experimental skills, and 3) innovative application. This lesson model was based on an idea of science education as a second language education. The children did not consider the introduction part of sharing scientific language to be difficult and their self-evaluation of keenness to learn was high. In the second part which involved practicing scientific methods and experimental skills, the difficulty level of the lesson showed an increase in their self-evaluation. It seemed that self-confidence also increased slightly at this stage. In the final stage involving innovative application, the children designed their original experiments, and discussed what was new in their findings. In the final stage, the self-evaluation of difficulty of the lesson further increased while their self-confidence and self-evaluation of keenness to learn rose as well. In conclusion, primary students can consistently and intentionally manipulate ‘scientific language and metaphor’, confidently face difficult questions and discuss something new about their findings collaboratively.
Eva Trnova
Masaryk University, Czech Republic
Title: How to Motivate Students to Learn Chemistry
Time : 10:00-10:30

Biography:
Eva Trnova works as senior lecturer at the Faculty of Education MU in the Czech Republic. She has completed her PhD in chemistry education. She has extensive experience with innovative methods of teaching chemistry. She has been engaged in chemistry education for a long time and she has published more than 20 papers in journals and has been serving as an editorial board member of journal dealt with education. She is a member of International Advisory Board in several conferences. She has been involved in many international projects dealing with the development of science education (e.g. PROFILES Project of SFP).
Abstract:
Current chemistry is developing rapidly and strongly affects human everyday lives. For this reason, chemistry is considered to be an important part of education for the current and future population. There is also expert consensus that science education (including chemistry) should be a compulsory part of the education of all children. But according to research findings (for example PISA) chemistry belongs to the least favourite school subjects. Because the socio-economic success of Europe 2020 depends on education of new generation, new ways of chemistry teaching/ learning which should prepare today’s children for adult roles as citizens, employees, managers, entrepreneurs, and parents, are sought. So a very important question arises how to motivate students to learn chemistry. In the presentation the most important factors influencing student interest in chemistry and ways how to support student engagement in learning of chemistry will be presented. Big attention will be paid to the innovative teaching methods of chemistry. Because the quality of teachers is one of the most important factors influencing the quality and effectiveness of chemistry education and student performance innovative ways of teacher education will be discussed.
Eva Trnova
Masaryk Unversity, Czech Republic
Title: Changes in Science Education for the 21st Century

Biography:
Eva Trnova works as senior lecturer at the Faculty of Education MU in the Czech Republic. She has completed her PhD in chemistry education. She has extensive experience with innovative methods of teaching chemistry. She has been engaged in chemistry education for a long time and she has published more than 20 papers in journals and has been serving as an editorial board member of journal dealt with education. She is a member of International Advisory Board in several conferences. She has been involved in many international projects dealing with the development of science education (e.g. PROFILES Project of SFP).
Abstract:
Science education including chemistry is undergoing changes due to its increasing importance these days, as it faces economic and social challenges. It is possible to register these changes in most European countries as well as the USA. Society requires to prepare the younger generation for the 21st century. We need a workforce with generally higher levels of STEM (Science, Technology, Engineering and Mathematics) literacy, as well as a sufficient number of highly gifted individuals entering scientific and engineering careers. To carry out these requirements it is necessary to change way of education and to find its appropriate content. Experts are trying to define a new paradigm of science education. But in order to be successful, important curricular changes have to be accepted by all of the stakeholders in education: students, their parents, politicians and especially by teachers, who should implement these curricular changes into practice. We will present research findings of the Czech Republic concerning the identification of views on science education, what opinions on current science education are held by stakeholders and what priority should be preferred in their opinion. The appropriate content of school chemistry will be discussed.
Esther S. Rösch
Pforzheim University of Applied Sciences, Germany
Title: Understanding Photography as Applied Chemistry: Using Talbot’s Calotype Process To Introduce Chemistry to Design Students
Time : 10:30-11:00

Biography:
Esther S. Rösch has completed her PhD from Karlsruhe Institute of Technology (KIT) in 2009. After graduation she worked as group leader and manager in the pharmaceutical industry. In 2013 she was appointed Professor of Bioanalytics in the Medical Engineering course of studies at Pforzheim University of Applied Sciences. Her educational goal is to inspire students with interdisciplinary projects in chemistry.
Silke Helmerdig is an artist and researcher working with and on photography. She holds a PhD from Goldsmiths College and is professor of Art Photography in the School of Design at Pforzheim University.
Abstract:
Generations of teachers and lecturers of chemistry have aimed at stimulating the curiosity of their students. Kindling an interest in chemistry for nonchemistry majors such as design students is even more of a challenge. Traditional photographic processes such as William Henry Fox Talbot’s calotype process are a link between the artistic and scientific disciplines. In 2−3 day workshops, design students without a major background in chemistry were able to define a reproducible protocol for Talbot’s gallic acid containing calotype process. The aim was to offer students the possibility to discover the chemical process on their own and to translate the procedure into creative artwork. With the experimental concept presented herein, students can be taught to approach an issue in a systematic way, to practice their problem solving skills, and to experience chemistry in a hands-on learning environment. Due to the workshop setup students can be coached individually in accordance with their progress. They can understand the chemical process, manipulate it, and use it in an artistic fashion. However, the molecular interpretation of a photograph is the means to an end. Photography is a well-known, ubiquitous process, and even today, young students are fascinated by the moment when the picture becomes visible in the dark room. Labor intensive photographs are appreciated in a different way than images taken with digital cameras or smartphones. Students without a chemical background succeeded in formulating a reproducible protocol for the calotype process and were able to pass on their knowledge to fellow students.
David J. Merkler
University of South Florida, USA
Title: Cellular Communication: Novel Endocannabinoid-like Lipids, Fruit Flies and Other Insects, N-Acyltransferases, and Subtraction Lipidomics
Time : 11:20-11:50

Biography:
David J. Merkler completed his Ph.D. at the age of 27 from the Pennsylvania State University in 1985 and completed postdoctoral studies in Dr. Vern L. Schramm's group at the Albert Einstein College of Medicine. Next, David took a position as a Senior Scientist at Unigene Laboratories, Inc. and contributed to the project on the in vitro production calcitonin. David has served on the Chemistry faculty at Duquesne University and the University of South. David’s academic research has been supported by the National Institutes of Health and he has 75 publications in reputable journals.
Abstract:
Fatty acid amides are an extensive family of cell signaling lipids with the general structure of R-CO-NH-Y. This structural simplicity belies a wealth of diversity amongst this lipid family as the R-group is derived from fatty acids (R-COOH) and the Y-group is derived from a number of biogenic amines (H2N-Y). The fatty acid amide family is divided into different classes, which are defined by parent amines. Examples include the N-acylethanolamines (NAEs, R-CO-NH-CH2-CH2OH), the N-acylglycines (NAGs, R-CO-NH-CH2-COOH), and the fatty acid primary amides (PFAMs, R-CO-NH2). In addition to the NAEs, the NAGs, and the PFAMs, other classes of fatty acid amides are known. As the best known fatty acid amide is N-arachidonoylethanolamine (anandamide), a fatty acid amide found in the human brain that binds to the cannabinoid receptors.
The Merkler laboratory has had a long interest in the fatty acid amides, with a focus on their biosynthesis. We and others have demonstrated that the NAGs and the NAEs are precursors to the PFAMs and were the first to identify N-oleoylglycine from a mammalian source, long-chain N-acylserotonins from Drosophila, and have characterized a set of N-acyltransferases from mammals, Drosophila melanogaster, the silkworm (Bombyx mori), and the red flour beetle (Tribolium castaneum). The fatty acid amide field is littered with many unanswered questions, including (a) have all the naturally-occurring fatty acid amides been uncovered, (b) which enzymes are involved in fatty acid amide metabolism in vivo, and (c) what are the respective contributions made by divergent pathways of fatty acid amide metabolism.
Arnos Arshaki Hovhannisyan
National Academy of Sciences of Republic of Armenia, Armenia
Title: Growing profi le of single crystals using polymer materials
Time : 11:50-12:20

Biography:
Abstract:
Oda Dahlen
Norwegian university of science and technology, Norway
Title: Monitoring the level of knowledge of learners to tailor web-based exercises at an individual level
Time : 12:20-12:50

Biography:
Abstract:
There is evidence in the literature that novice learners benefit from direct instructional methods while more experienced learners benefit from a more discovery-based approach. When a task efficient for novice learners becomes inefficient for experienced learners, it is called the expertise reversal effect, and illustrates the importance of being able to tailor a exercise to the level of the learner. This becomes especially relevant with the increasing use of web-based learning, where it is possible to tailor every small step of the learning process giving each individual a unique learning experience. To do this, we need a continuous monitoring of the level of knowledge of the learner. We designed a self-monitoring test to see if the students were able to evaluate their own level of knowledge, and a rapid science-based test in a specific area of science. We compared both tests with traditional measures of knowledge.
Hasmik N. Khachatryan
Institute of Organic Chemistry, Scientific-Technological Center of Organic and Pharmaceutical Chemistry NAS RA, Armenia
Title: The synthesis of exocyclic derivatives of pyrazoles based on 1H-pyrazolylbutannitriles
Time : 12:50-13:20

Biography:
Hasmik Khachatryan received her master degree at Chemical faculty of Yerevan State University in 2014 under supervision of L. Galstyan. Now she is PhD student since 2015 under supervision of S. Hayotsyan and professor H. Attaryan. During her scientific work she has already done a lot of experiments and has 8 published articles. Her research interest is aza-Michael reaction through azoles in free solvent, free catalyst systems. She participated in 4th International Conference of Young Scientists “Chemistry Today-2014”, Yerevan, Armenia 18-22, 2014 and 2nd European Organic Chemistry Congress, Amsterdam, Netherlands 02-03, 2017.
Abstract:
The exocyclic derivatives of pyrazoles are included in the structure of many drugs, both natural and synthetic [1-5]. Thus, considering the importance of obtaining exocyclic pyrazole derivatives, it is a key factor to study various transformations of chemically suitable nitrilpyrazoles (1) according to the following scheme:
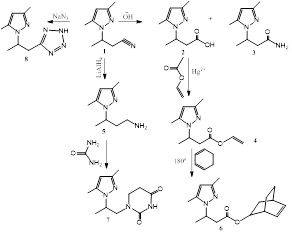
The structure of obtained products were confirmed by 1H NMR, 13C, IR spectroscopy and data of elemental analysis.
